by Cnespral | Sep 28, 2015
Restraint system GENERAL INFORMATION ABOUT THE PRODUCT This product is manufactured on the basis of the quality criteria set out in Real Decreto 414/96, transposing Directive 93 / 42EEC on medical devices. It also meets all the essential requirements applicable to it....

by Cnespral | Sep 28, 2015
Immobilizers GENERAL INFORMATION ABOUT THE PRODUCT This product is manufactured on the basis of the quality criteria set out in Real Decreto 414/96, transposing Directive 93 / 42EEC on medical devices. It also meets all the essential requirements applicable to it....
by Cnespral | Sep 28, 2015
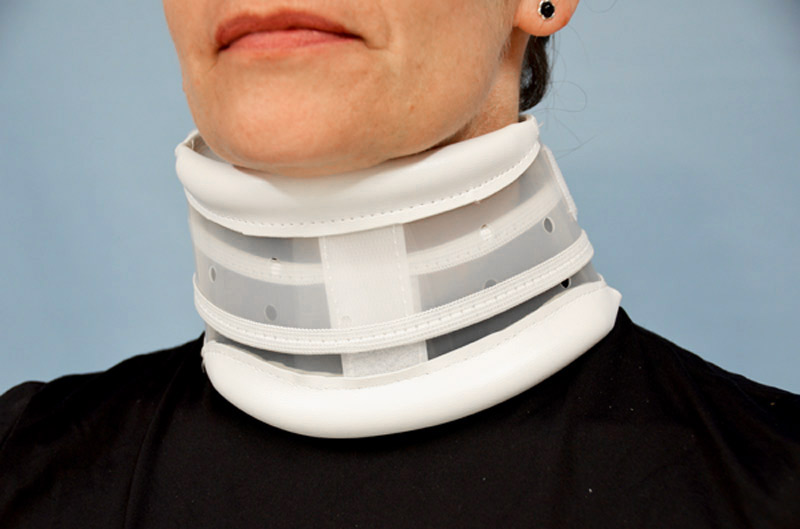
Collars GENERAL INFORMATION ABOUT THE PRODUCT This product is manufactured on the basis of the quality criteria set out in Real Decreto 414/96, transposing Directive 93 / 42EEC on medical devices. It also meets all the essential requirements applicable to it....

by Cnespral | Sep 28, 2015
Slings INDICATIONS Download the shoulder and collarbone, inflammatory processes which require restraint, clavicle and humerus fractures, holding the arms in casts, sprains and dislocations of the upper limb. CHOOSING THE SIZE Choice of size is vital to this product...

by Cnespral | Sep 28, 2015
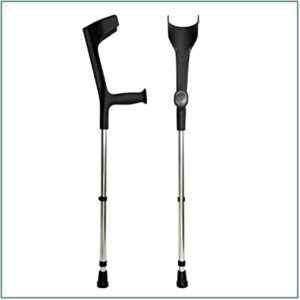
Orthopedic crutches GENERAL INFORMATION ABOUT THE PRODUCT All our rods are made of anodized aluminum tube silver with 1.5 mm. Thickness and height adjustable by metal stent. The handles and elbow pads are made of polypropylene. The ferrule is P.V.C. with draining...